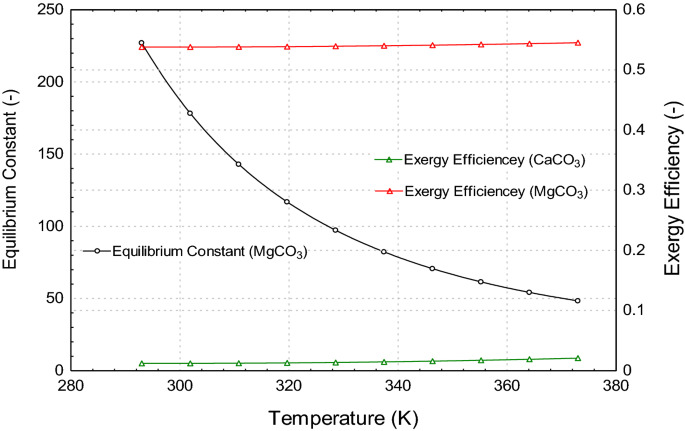
Thermodynamic analysis of theoretical dolomite formation from seawater and captured carbon dioxide | SpringerLink
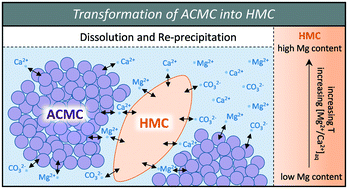
Effect of temperature on the transformation of amorphous calcium magnesium carbonate with near-dolomite stoichiometry into high Mg-calcite - CrystEngComm (RSC Publishing)

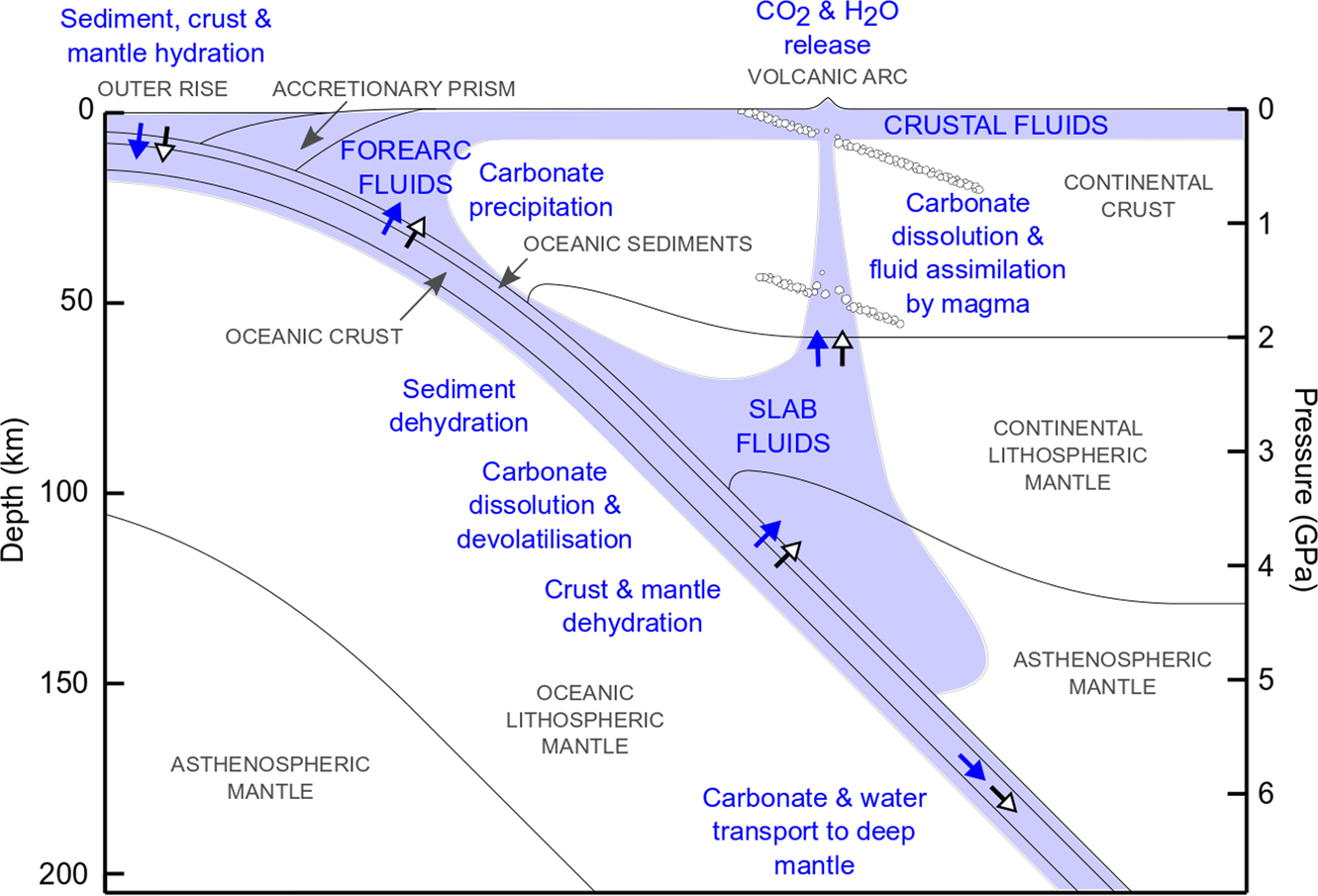
Mineralogy, nucleation and growth of dolomite in the laboratory and sedimentary environment: A review - Gregg - 2015 - Sedimentology - Wiley Online Library
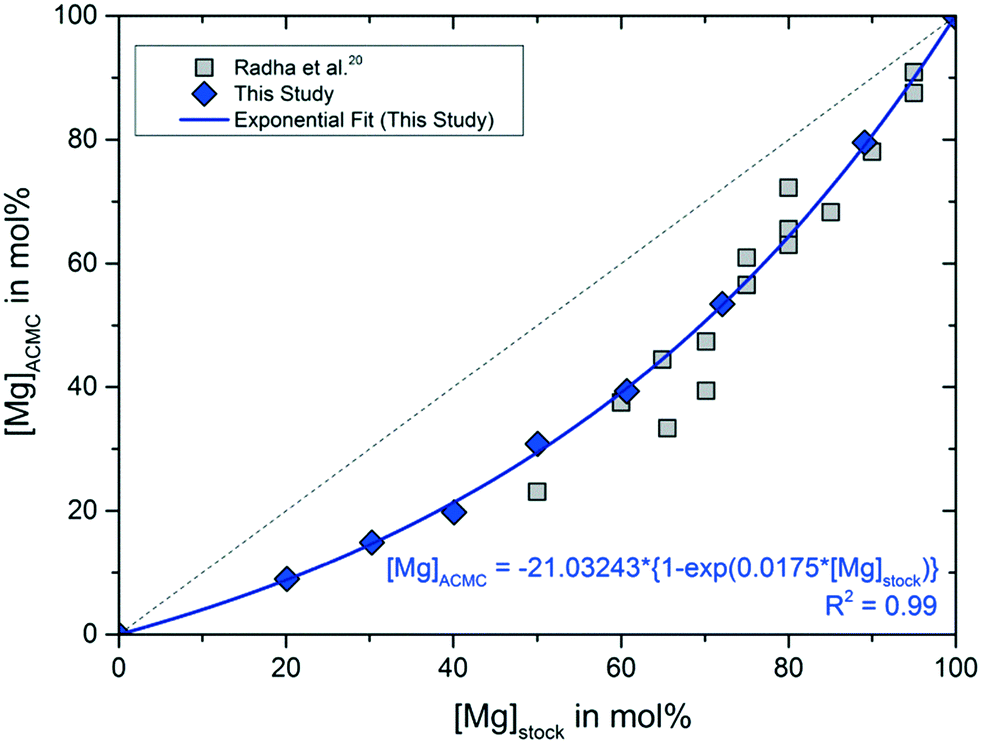
Solubility investigations in the amorphous calcium magnesium carbonate system - CrystEngComm (RSC Publishing) DOI:10.1039/C8CE01596A
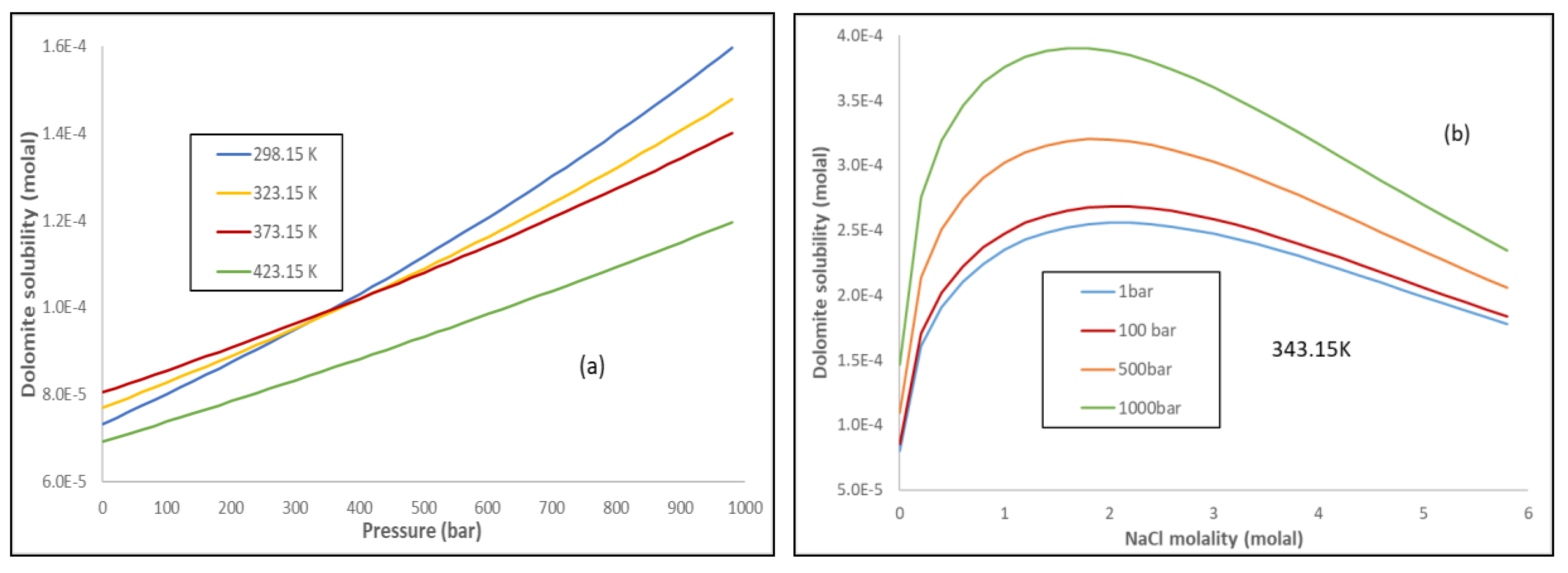
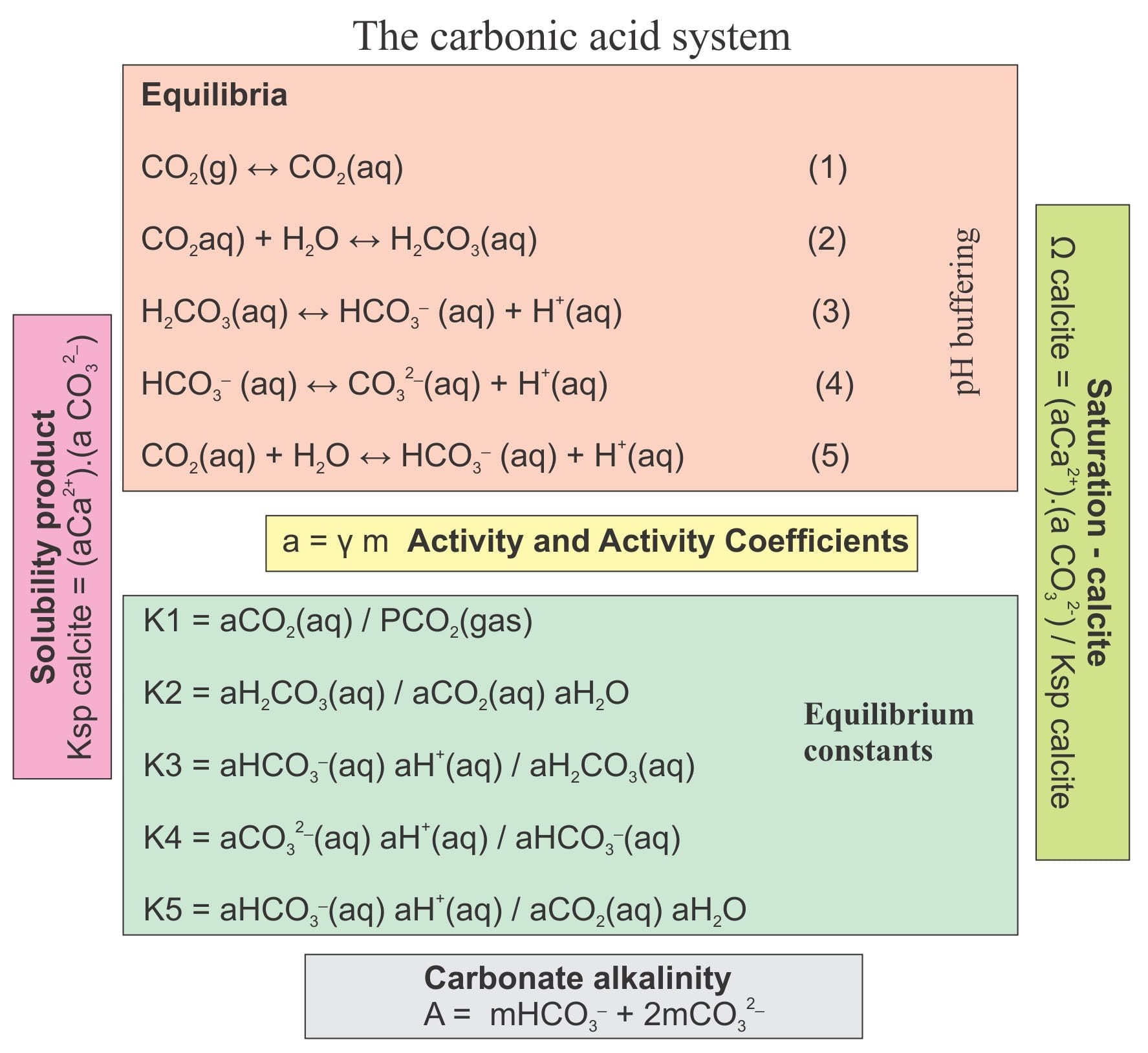
Energies | Free Full-Text | Thermodynamic Modeling of CO2-N2-O2-Brine-Carbonates in Conditions from Surface to High Temperature and Pressure

Saturation state of calcite and dolomite as a function of pH in the... | Download Scientific Diagram

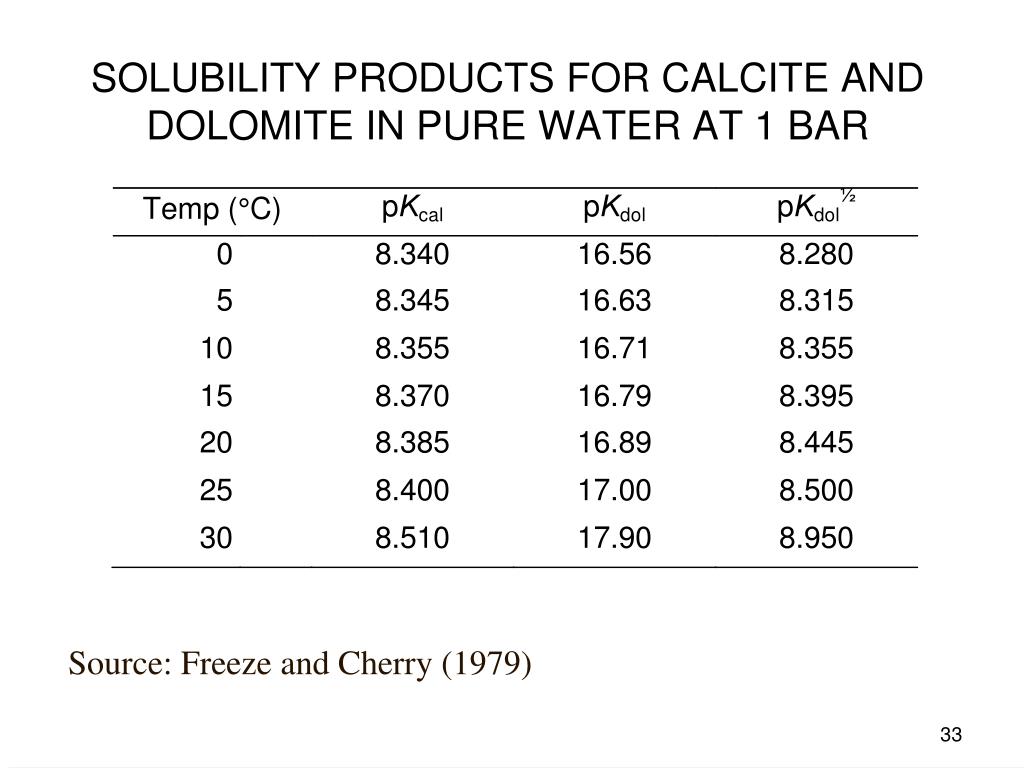
Solubility product constants for natural dolomite (0–200 °C) through a groundwater-based approach using the USGS produced water database | American Journal of Science

Greenhouse conditions induce mineralogical changes and dolomite accumulation in coralline algae on tropical reefs | Nature Communications

Calcite, dolomite and magnesite dissolution kinetics in aqueous solutions at acid to circumneutral pH, 25 to 150 °C and 1 to 55 atm pCO2: New constraints on CO2 sequestration in sedimentary basins - ScienceDirect
Difficulties are often encountered in processing heterogeneous mineral systems under conditions predicted on the basis of single

Calcite, dolomite and magnesite dissolution kinetics in aqueous solutions at acid to circumneutral pH, 25 to 150 °C and 1 to 55 atm pCO2: New constraints on CO2 sequestration in sedimentary basins - ScienceDirect